
Click Therapeutics announced that CT-132 met its primary endpoint in the phase 3 ReMMi-D trial.

Click Therapeutics announced that CT-132 met its primary endpoint in the phase 3 ReMMi-D trial.

Results from the PALISADE study were published in The New England Journal of Medicine.
The protein-based vaccine includes a monovalent component corresponding to the Omicron variant JN.1 strain.
Catch up on important immunization news from the month of August.
Chijioke Bennett, executive director of clinical development at Novavax, discusses how health care providers can increase awareness around the importance of COVID-19 vaccines.

Checkout these important FDA updates from the month of August 2024.
Results from the INTEGUMENT-OLE phase 3 trial showed the therapy was also well-tolerated, with no new safety signals observed during treatment of up to 56 weeks.

Tris Pharma will examine the efficacy and safety of cebranopadol in the ALLEVIATE-1 and ALLEVIATE-2 studies.

Check out this list of our top 10 stories from August 2024.

Chijioke Bennett, executive director of clinical development at Novavax, discusses how health care providers can increase awareness around COVID-19 vaccination and how to help patients overcome vaccine hesitancy.

Check out these top featured stories from Drug Topics in August 2024.

The Stelo glucose biosensor system is intended for use in adults who do not use insulin or in those without diabetes who wish to better understand how diet and exercise affect blood glucose levels.

Check out these featured Drug Topics interviews from August 2024.

Check out important updates from the FDA for the week of August 19.

Recent research on diabetes has examined its bidirectional relationship with mental health, the significant increase in prevalence over the last decade, and how both diet and exercise can help prevent type 2 diabetes.

Increased incidence of typed 2 diabetes was associated with higher consumption of processed meat, unprocessed red meat, and poultry.
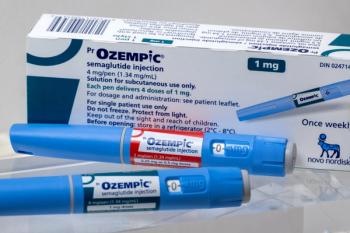
Timothy Mackey, MAS, PhD, discusses a study that sought to determine the risk of sourcing semaglutide from online platforms.

Timothy Mackey, MAS, PhD, discusses the dangers associated with sourcing GLP-1s from illegal online pharmacies and the key role pharmacists play in warning patients.

The approval of apremilast to treat plaque psoriasis in children and adolescents aged 6 years and older was based on data from the phase 3 SPROUT trial.

Focus group participants were receptive to using eHealth tools, but noted several barriers such as cost and availability.

Check out important updates from the FDA for the week of August 12.
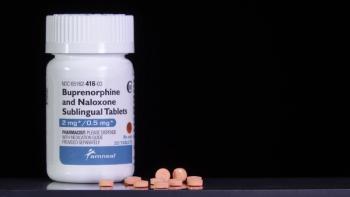
Community pharmacies in counties with high racialized economic segregation had higher odds of restricting buprenorphine.
Izokibep from Affibody is a small therapeutic protein designed to inhibit IL-17A.

Medtronic also announced it has entered a global partnership with Abbott in an effort to expand CGM device options for patients living with diabetes.

Rejoyn, developed by Otsuka Pharmaceutical and Click Therapeutics, is only accessible with a prescription from a health care provider.

Two phase 3 trials evaluating nemolizumab found that 41% of patients treated with the therapy achieved at least a 4-point reduction in itch intensity at week 16.

Ascendis Pharma said it is currently completing manufacturing of commercial product, which it anticipates will be available in the US in the first quarter of 2025.

The agency said it could not approve the new drug application based on the submitted data and requested an additional Phase 3 trial to “further study the safety and efficacy” of the therapy.

Check out important updates from the FDA for the week of August 5.

Sam Clark, MD, PhD, founder and CEO of Terran Biosciences, discusses the implications of a potential FDA approval of MDMA-assisted therapy for PTSD.