The Institute for Clinical and Economic Review assessed the formularies of 11 payers, covering 57 million people, to determine access to drugs that the organization had reviewed for cost effectiveness in 2022.

The Institute for Clinical and Economic Review assessed the formularies of 11 payers, covering 57 million people, to determine access to drugs that the organization had reviewed for cost effectiveness in 2022.
Findings from the study conducted by 3 Axis Advisors confirm that PBM practices have an impact on pharmacies.

After semaglutide and tirzepatide were approved, and ozempic use continues to rise, Medicare spending is also increasing because of a high demand for GLP-1 therapies.

Positive results from the FLOW trial demonstrate the potential for Ozempic to become the first GLP-1 treatment option for people living with type 2 diabetes and chronic kidney disease.

The new approval is supported by the phase 3 Study 4030, which evaluated the efficacy, safety, and tolerability profile of Biktarvy in a broad range of people with HIV-1 with or without pre-existing NRTI resistance, including those with the M184V/I resistance.

Large pharmacy benefit managers have switched up their coverage of biosimilars this year—especially for biosimilars to adalimumab.
Innovation in the vaccine area continues. In 2023, the FDA approved six vaccines, including several important firsts. But they face a difficult landscape where federal policies dictate coverage.

The Affordable Care Act mandates that contraceptives must be covered by health plans and insurers without cost-sharing.

The Congressional Budget Office last year estimated that price negotiation will lower average drug prices in Medicare and will reduce the budget deficit by $25 billion in 2031, including lowering Part D spending by $14 billion and Part B spending by $9 billion. Other federal spending will be lowered by $1 billion.

Through interactive lessons, skill-building modules and weekly goal setting and tracking, AspyreRx allows patients to connect changes in behavior to improvements in blood sugar and other biometrics.
In 2023, the FDA approved six vaccines, including 2 for respiratory syncytial virus (RSV), and the first vaccine to prevent the mosquito-borne virus chikungunya.

Despite affecting 13 million Americans annually, existing post-traumatic stress disorder (PTSD) treatments offer modest relief and more effective approaches are needed.

According to a presentation at AMCP Nexus 2023, a pharmacist-led program initiated by the Mayo Clinic improved access and lowered costs for patients by promoting biosimilar adoption.
During a session at AMCP Nexus 2023, presenters discussed the delicate balance between cost and innovation as it relates to forthcoming oncology treatments.

Disparities for patients with HIV who have cancer exist, and this is driven by social and structural determinants of health, as well as HIV stigma and lack of training for oncologists.
Abacavir/dolutegravir/lamivudine is a fixed-dose combination containing two nucleoside reverse transcriptase inhibitors (NRTIs) and integrase strand transfer inhibitor (INSTI).

Over the last two legislative sessions, many bills have been introduced in the both the House and the Senate, but there are four bills that are important to watch.
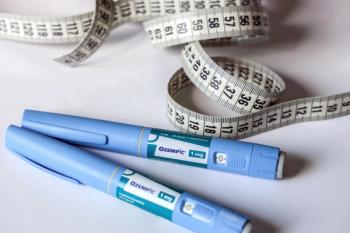
Drugs like Ozempic and Wegovy, two semaglutide products made by Novo Nordisk, as well as Mounjaro (tirzepatide), mimic a hormone called glucagon-like peptide-1 (GLP-1) that targets areas of the brain that regulate appetite and food intake.
The clinical pharmacist will play an important role as a medication expert, filling in for the role that pharmaceutical manufacturers once did in supporting patients and prescribers.
Over the last 5 years, both PBMs and drug manufacturers have implemented restrictions that raise patients’ cost burden for specialty medications.

A Kaiser Family Foundation analysis found the 10 drugs accounted for more than half of the increase in gross Part D spending from 2018 to 2021.

Three new bills signed Monday will require PBMs to use prescription drug rebates to lower premiums and out-of-pocket costs for consumers and prevent the practice of spread pricing.

The median annual cost for new oncology medicines launched in 2022 was $260,000, up from $63,534 10 years ago, an IQVIA report finds.

Members of the United States Senate Committee on Finance want to modernize the rules around PBM business practices to lower out-of-pocket costs and increase competition.

Members of the Senate Finance Committee are pushing PBMs to modernize the rules regarding out of pocket costs and competition.

The suit alleges that Express Scripts, Prime and Prime customer Humana Pharmacy Solutions are able to share drug pricing and rebate information to increase prices for insulins, biologics and cancer drugs.

If approved, Perrigo’s Opill would be the first nonprescription birth control pill ever.

The drug Leniolisib, now with the brand name Joenja, treats APDA, a genetic disorder that impairs the immune system. Its annual list price will be $547,500.

The proposed legislation, sponsored by Chuck Grassley and Maria Cantwell, aims to necessitate transparency from PBMs.

Per VA regulations, anyone who has had a stroke or seizure within the last year, as well as those with MRIs showing microhemmorrhages, aneurysms, lesions or tumors would be excluded from coverage of the drug, called Leqambi

Published: September 13th 2023 | Updated:

Published: January 24th 2024 | Updated:

Published: March 30th 2023 | Updated:

Published: June 1st 2023 | Updated:

Published: November 7th 2023 | Updated:
Published: September 12th 2023 | Updated: