Shingrix Guidelines for the Immunocompromised
The CDC has updated recommendations for shingles vaccination.
The CDC has updated1 its clinical considerations for the use of Shingrix (recombinant zoster vaccine; RZV) to help prevent shingles and related complications in immunocompromised adults aged 19 and older. The move follows the recent unanimous recommendation of the vaccine by the agency’s Advisory Committee on Immunization Practices (ACIP) for adults who are or who will be immunodeficient or immunosuppressed due to disease or therapy.
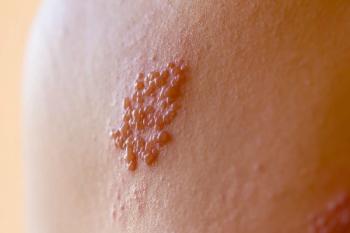
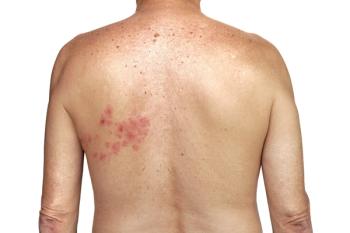
Shingles (herpes zoster; HZ) is caused by the varicella zoster virus (VZV), which initially causes chickenpox. The virus then remains inactive in the body until it reactivates decades later as shingles. It is estimated that virtually all Americans born before 1980 have had chickenpox and are consequently at risk for shingles.
According to ACIP reports2 there are approximately 7 million immunocompromised adults in the United States, including 3 million stem cell transplant recipients, patients with hematologic or solid tumor malignancies, solid organ transplant recipients, and people living with HIV. Another 22 million Americans are living with autoimmune and/or inflammatory conditions like rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease (IBD). All are at increased risk for developing shingles.
“Risk of herpes zoster (HZ), severe disease, and complications [is] generally higher in immunocompromised (IC) populations,” ACIP noted. “RZV can potentially address an unmet need for HZ prevention in IC populations.”
The CDC’s new clinical considerations for Shingrix and immunocompromised adults include:
- Persons who are or will be immunodeficient or immunosuppressed and would benefit from completing the Shingrix 2-shot series in less than the recommended 2- to-6-month period may receive the second dose 1 to 2 months after the first. “A shorter interval between doses may facilitate avoiding vaccination during periods of more intense immunosuppression,” the CDC noted.
- Patients should be vaccinated before becoming immunosuppressed. If this is not possible, vaccination should be timed for when the immune response is likely to be most robust. The general level of immune competence in a patient can be judged by a number of factors including disease severity and duration, clinical stability, complications and comorbidities, and any potentially immunosuppressing treatment.
- Shingrix, like other recombinant and adjuvanted vaccines, can be administered at the same time as other vaccines, including the flu vaccine.
- Evidence of immunity to varicella, which confirms the need for RZV, can be determined 3 ways: through documentation of 2 doses of chickenpox vaccine, or laboratory evidence of immunity or laboratory confirmation of the disease, or diagnosis/verification of a history of varicella or HZ by a healthcare provider.
- Providers should counsel immunocompromised patients about expected local and system side effects of the vaccine prior to vaccination so they know what to expect.
- The ACIP currently does not recommend RZV for use during pregnancy, so providers are advised to delay the vaccine until post pregnancy. “Recombinant vaccines such as RZV pose no known risk for mothers who are breastfeeding or their infants,” the CDC states. “Clinicians may consider vaccination without regard to breastfeeding status if RZV is otherwise indicated.”
- History of severe allergic reaction to any component of the vaccine is a contraindication for vaccination. Moderate or severe acute illness with or without fever should delay vaccination until symptoms conclude. This includes a current episode of HZ.
References
- Clinical considerations for use of recombinant zoster vaccine (RZV, Shingrix) in immunocompromised adults aged ≥19 years. CDC. Reviewed January 20, 2022. Accessed April 15, 2022.
- Anderson T. Zoster vaccines session: Burden of herpes zoster in immunocompromised adults. National Center for Immunization & Respiratory Diseases. Division of Viral Diseases, Viral Vaccine Preventable Diseases Branch. June 25, 2021. Accessed April 15, 2022.
Newsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.