Patients With Cancer at Greater Risk for Shingles
Shingles vaccination can protect against infection and complications.
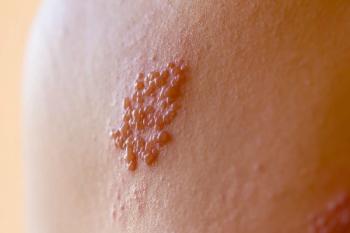
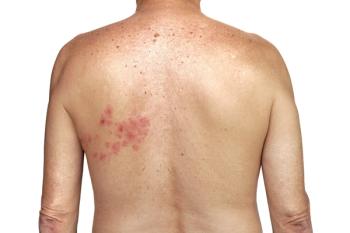
Shingles (herpes zoster, HZ) is an opportunistic infection that occurs when the latent varicella zoster virus (VZV) reactivatesand the body’s immune system is unable to fight it off. Two primary reasons for this are advanced age and immunosuppression due to illness or medical treatments. Cancer patients often fall into both of those categories and are at greater risk of developing shingles and experiencing more severe cases and complications.
A study published in The Journal of Infectious Diseases1 examined the risk of shingles before and after a new cancer diagnosis across a range of cancer types among approximately 240,000 adults in Australia. Researchers found that a cancer diagnosis of any kind was associated with about a 40% increase in risk for developing shingles compared to the risk in someone without cancer. Patients with hematological cancers had a more than 3-fold higher risk of developing shingles, while patients with solid tumor cancers had a 30% higher shingles risk.
“For hematological cancer, increases in zoster risk are apparent in the 2 years preceding diagnosis and treatment; for solid organ cancers, the increased risk appears to be largely associated with receipt of chemotherapy,” the study authors concluded. “Zoster vaccination now holds promise as a preventive strategy considered for cancer patients, particularly those expected to receive chemotherapy.”
A second study, conducted in the United States and published in Clinical Infectious Diseases,2 examined the risk of HZ and associated complications in several categories of immunocompromised adults, including those with cancer. Researchers of that study concluded that “HZ was common among all immunocompromised populations studied, exceeding the expected HZ incidence among immunocompetent adults aged ≥60 years.”
Protection against shingles is important for those with cancer—but not necessarily because shingles makes their cancer worse or prevents treatment, says Dale Shepard, MD, a medical oncologist at Cleveland Clinic.
“You’re looking at a population that likely has symptoms of their disease, and they may have symptoms of their treatments,” Shepard told Drug Topics®.“We don’t want people to have cancer, to have cancer treatments, to have those complications, and then deal with shingles as well.”
Shepard encourages his patients to include shingles vaccination as part of an overall immunization strategy. “We like to make sure that they’ve gotten a pneumonia vaccine if they’re eligible, we like to make sure they’ve had an influenza vaccine,” he said. “We make sure that the major health concerns are addressed, that they’ve covered the important health maintenance topics.”
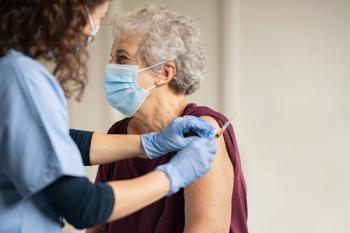
The first line of defense against shingles for all at-risk individuals is vaccination with Shingrix, approved by the FDA in late 2017 for immunocompetent adults 50 years and older. It has been proven in clinical trials to be over 90% effective in preventing shingles.
The FDA recently expanded its approval of Shingrix to include immunocompromised adults 18 years and older based on clinical studies of the vaccine’s safety and efficacy in patients who had undergone an autologous hematopoietic stem cell transplant or treatment for hematological malignancies. Data on patients with solid tumors was also considered.
The CDC Advisory Committee on Immunization Practices has also recommended the use of Shingrix in adults 19 years and older who are or will be immunodeficient or immunosuppressed due to disease or therapy. The recommendation is under review by the director of the CDC and the U.S. Department of Health and Human Services.
References
1. Kawai K, Yawn BP.
2. McKay SL, Guo A, Pergam SA, Dooling K.
Newsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.