ACIP Recommends Shingrix for Immunocompromised Adults
At-risk adults aged 19 and older may soon be eligible
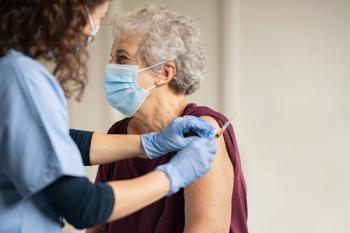
The CDC’s Advisory Committee on Immunization Practices (ACIP) has unanimously recommended the use of GlaxoSmithKline’s Shingrix vaccine in adults 19 years and older who are or will be immunodeficient or immunosuppressed due to disease or therapy.1 Currently recommended only for adults 50 and older, Shingrix helps to prevent
ACIP’s vote follows a decision earlier this year by the
The FDA and ACIP recommendations open up protection against shingles to millions of at-risk individuals in the United States who do not meet the current age requirement for the vaccine.
"The ACIP’s vote helps to address an existing unmet need as individuals who are immunocompromised are at an increased risk of the disease,” noted Sabine Luik, Chief Medical Officer & SVP Global Medical Regulatory & Quality at GSK, in a press release from the company.1
According to GSK, the ACIP recommendation is based on clinical studies examining the safety and efficacy of Shingrix in adults 18 and older who had undergone an autologous hematopoietic stem cell transplant and those undergoing treatment for hematological malignancies. Additional safety and immunogenicity data were generated in adults who were, or were anticipated to be, immunodeficient or immunosuppressed due to known disease or therapy. This includes patients with HIV, solid tumors, and renal transplants.
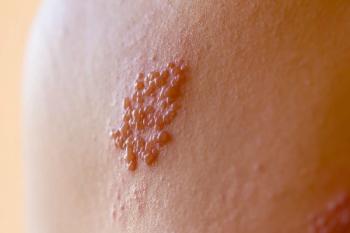
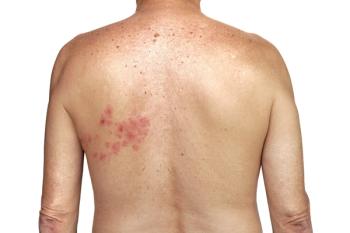
A primary infection by the
More than 99% of Americans over the age of 50 are infected with VZV from previous exposure, and 1 in 3 will develop shingles in their lifetime.3 The rate increases to 1 in 2 for adults 85 years and older. About 1 million Americans develop shingles annually.
In addition to a
In immunocompetent adults, Shingrix is administered in 2 doses delivered 2 to 6 months apart. In adults who are or will be immunodeficient or immunosuppressed and would benefit from a shorter vaccination schedule, the second dose can be administered 1 to 2 months after the first dose.
The ACIP recommendation has been forwarded to the director of the CDC and the U.S. Department of Health and Human Services for review and approval. Once approved, the final recommendations will be published in a future Morbidity and Mortality Weekly Report.
References
1. US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices votes unanimously to recommend Shingrix for immunocompromised adults aged 19 and up. News release. GSK. October 20, 2021. Accessed November 24, 2021.
2. Shingrix approved in the US for prevention of shingles in immunocompromised adults. News release. GSK. July 26, 2021. Accessed November 24, 2021.
3. Shingles (herpes zoster) burdens and trends. CDC. August 14, 2019. Accessed November 24, 2021. https://www.cdc.gov/shingles/surveillance.html
Newsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.