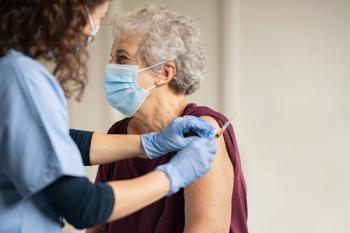
Shingles Vaccination Compliance and Implications for Multidose COVID-19 Vaccines
Investigators evaluated older adults’ compliance with the 2-dose shingles vaccine regimen.
The FDA authorization of the
The Kaiser Family Foundation (
“We were curious to know more about experience with other multidose vaccines, particularly among older adults who have really been hardest hit by COVID-19,” Juliette Cubanski, PhD, deputy director of the program on Medicare policy, KFF, told Drug Topics®. “Shingrix also requires 2 doses, so the Medicare Part D experience offered us a good opportunity to look at what the experience was among an older population with a multidose vaccine.”
Cubanski and her colleagues looked at Medicare beneficiaries who received an initial dose of Shingrix in the first half of 2018. They then analyzed what percentage received the second dose within the recommended timeframe of 2 to 6 months, and which subgroups of beneficiaries were more or less likely to receive both doses.1
“The majority of Medicare beneficiaries who received an initial dose of the Shingrix vaccine received the second dose within 6 months, but follow-up rates were lower among beneficiaries in communities of color, those who are younger than age 65 with long-term disabilities, and low-income beneficiaries,” the investigators summarized in a December 2020 press release.1
Specifically, Shingrix vaccination compliance rates were highest among White beneficiaries (76%), followed by Asian/Pacific Islander (69%), American Indian/Alaska Native (61%), Black (61%), and Hispanic (58%) beneficiaries.1
The analysis also revealed that beneficiaries with incomes less than 150% of poverty were less likely than beneficiaries with higher incomes to receive the second dose of the shingles vaccine within 6 months. Finally, Medicare beneficiaries under age 65 who qualify because of a long-term disability were less likely than beneficiaries aged 65 and older to receive a second dose of Shingrix within 6 months.1
“The fact that we did observe differences in the percent of beneficiaries who got the second dose depending on ethnicity, different age cohorts, and income status is worrisome because data shows that Blacks, Hispanics, and older adults have been hard hit by the pandemic,” Cubanski explained.
She noted that there are important differences between the
Another important difference is Shingrix is covered under Medicare Part D, which requires a co-payment from beneficiaries. The COVID-19 vaccines have no out-of-pocket costs for patients, potentially removing a financial barrier to access.1
“It will be important for trusted sources of information—and that could differ from 1 patient population to another—to communicate the importance of getting the vaccine and the need to get the follow-up dose,” Cubanski concluded. “Clearly, steps like getting people their second appointment at the time that they get their first dose makes a lot of sense.”
Reference
1. Cubanski J, Neuman T, Damico A. Who Didn't Get a Second Shingrix Shot? Implications for Multidose COVID-19 Vaccines. Last updated December 14, 2020. Accessed January 29, 2020. https://www.kff.org/medicare/issue-brief/who-didnt-get-a-second-shingrix-shot-implications-for-multidose-covid-19-vaccines/
Newsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.